exploring blood
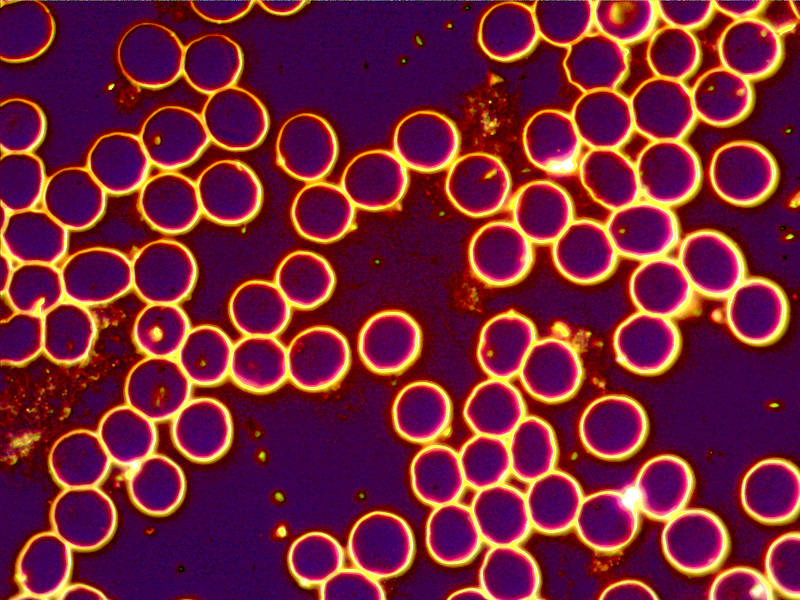
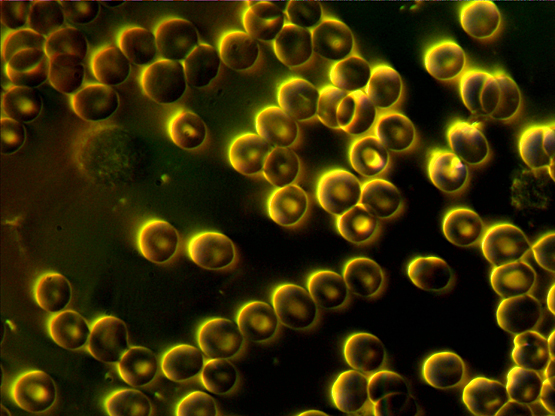
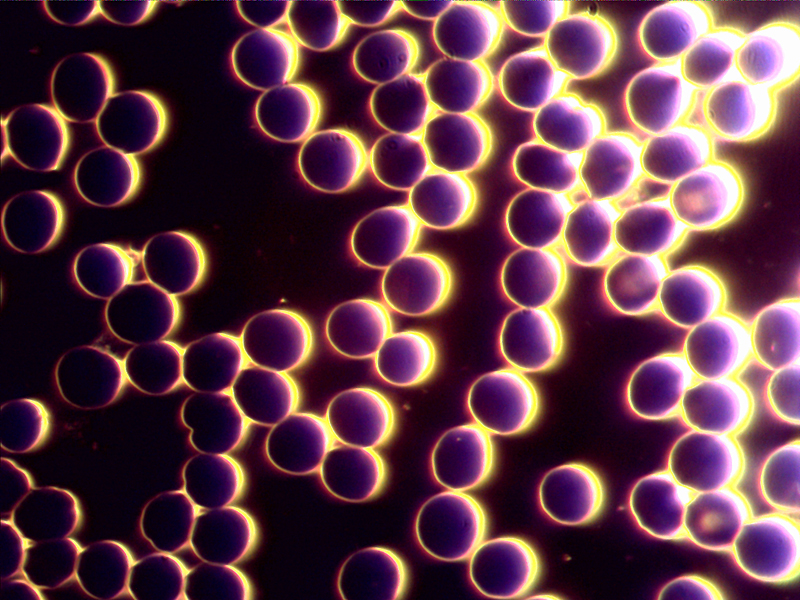
dec 12 – both live and dry blood
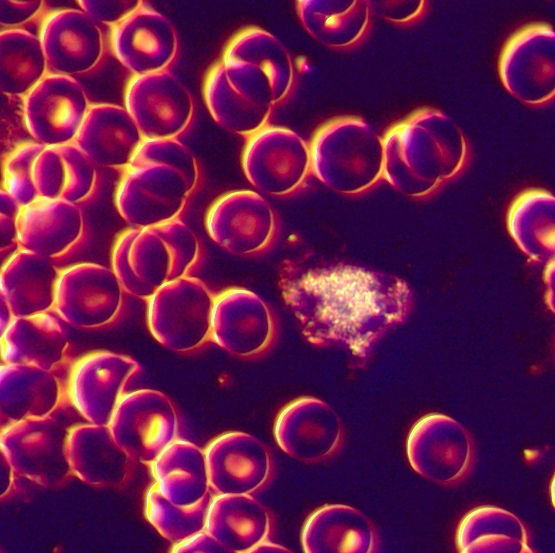
video on white blood cells
7 dry blood layers out of 8
oct 28 2012 – before going back to naples
oct 19th – friends
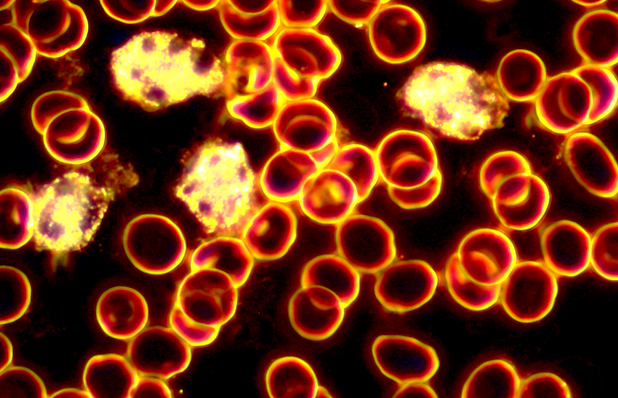
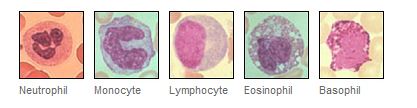
over view of the white blood cells
is this cholesterol in the blood???
agranulocytes / granulocytes
There are two types of agranulocytes:
1. Lymphocytes
2. Monocytes
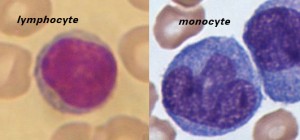 Lymphocytes are much more common in the lymphatic system, and include the so-called “natural killer T-cells”. The blood has three types of lymphocytes: B cells, T cells and natural killer cells (NK cells). B cells make antibodies that bind to pathogens to enable their destruction. CD4+ (helper) T cells co-ordinate the immune response (they are what becomes defective in an HIV infection). CD8+ (cytotoxic) T cells and natural killer cells are able to kill cells of the body that are infected by a virus. T cells are crucial to the immune response because they possess a unique ‘memory’ system which allows them to remember past invaders and prevent disease when a similar invader is encountered again.
Lymphocytes are much more common in the lymphatic system, and include the so-called “natural killer T-cells”. The blood has three types of lymphocytes: B cells, T cells and natural killer cells (NK cells). B cells make antibodies that bind to pathogens to enable their destruction. CD4+ (helper) T cells co-ordinate the immune response (they are what becomes defective in an HIV infection). CD8+ (cytotoxic) T cells and natural killer cells are able to kill cells of the body that are infected by a virus. T cells are crucial to the immune response because they possess a unique ‘memory’ system which allows them to remember past invaders and prevent disease when a similar invader is encountered again.
Monocytes share the “vacuum cleaner” (phagocytosis) function of neutrophils, but are much longer lived as they have an additional role: they present pieces of pathogens to T cells so that the pathogens may be recognized again and killed, or so that an antibody response may be mounted. Monocytes are also known as macrophages after they migrate from the bloodstream and enter tissue.
There are three types of granulocytes,
Granulocytes are a category of white blood cells characterized by the presence of granules in their cytoplasm
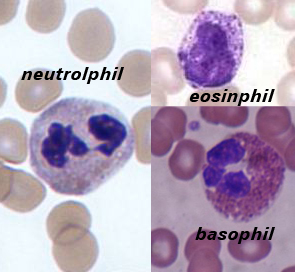 Neutrophils
Neutrophils
A neutrophil with a segmented nucleus (center and surrounded by erythrocytes), the intra-cellular granules are visible in the cytoplasm (Giemsa-stained high magnification)
Neutrophils are normally found in the bloodstream and are the most abundant type of phagocyte, constituting 50% to 60% of the total circulating white blood cells.[3] One litre of human blood contains about five billion neutrophils 5×109,[4] which are about 12-15 micrometers in diameter,[5] and live approximately 6 days.[5] Once neutrophils have received the appropriate signals, it takes them about thirty minutes to leave the blood and reach the site of an infection.[6] Neutrophils do not return to the blood; they turn into pus cells and die.[6] Mature neutrophils are smaller than monocytes, and have a segmented nucleus with several sections(two to five segments); each section is connected by chromatin filaments. Neutrophils do not normally exit the bone marrow until maturity, but during an infection neutrophil precursors called myelocytes and promyelocytes are released.[7]
Neutrophils have three strategies for directly attacking micro-organisms: phagocytosis (ingestion), release of soluble anti-microbials (including granule proteins), and generation of neutrophil extracellular traps (NETs).[8] Neutrophils are professional phagocytes[9]: they are ferocious eaters and rapidly engulf invaders coated with antibodies and complement, and damaged cells or cellular debris. The intra-cellular granules of the human neutrophil have long been recognized for their protein-destroying and bactericidal properties.[10] Neutrophils can secrete products that stimulate monocytes and macrophages; these secretions increase phagocytosis and the formation of reactive oxygen compounds involved in intracellular killing.[11] Neutrophils have two types of granules; primary (azurophilic) granules (found in young cells) and specific granules (which are found in more mature cells). Primary granules contain cationic proteins and defensins that are used to kill bacteria, proteolytic enzymes and cathepsin G to break down (bacterial) proteins, lysozyme to break down bacterial cell walls, and myeloperoxidase (used to generate toxic bacteria-killing substances).[12] In addition, secretions from the primary granules of neutrophils stimulate the phagocytosis of IgG antibody-coated bacteria.[13] The secondary granules contain compounds that are involved in the formation of toxic oxygen compounds, lysozyme, and lactoferrin (used to take essential iron from bacteria).[12] Neutrophil extracellular traps (NETs) comprise a web of fibers composed of chromatin and serine proteases that trap and kill microbes extracellularly. Trapping of bacteria is a particularly important role for NETs in sepsis, where NET are formed within blood vessels.[14]
Eosinophils
Eosinophils also have lobed nuclei (two to four lobes). The number of granules in an eosinophil can vary because they have a tendency to degranulate while in the blood stream.[15] Eosinophils play a crucial part in the killing of parasites (e.g., enteric nematodes) because their granules contain a unique, toxic basic protein and cationic protein (e.g., cathepsin[12]);[16] receptors that bind to IgE are used to help with this task.[17] These cells also have a limited ability to participate in phagocytosis,[18] they are professional antigen-presenting cells, they regulate other immune cell functions (e.g., CD4+ T cell, dendritic cell, B cell, mast cell, neutrophil, and basophil functions),[19] they are involved in the destruction of tumor cells,[15] and they promote the repair of damaged tissue.[20] A polypeptide called interleukin-5 interacts with eosinophils and causes them to grow and differentiate; this polypeptide is produced by basophils.[16]
Basophils
Basophils are one of the least abundant cells in bone marrow and blood (occurring at less than two percent of all cells). Like neutrophils and eosinophils, they have lobed nuclei; however, they have only two lobes, and the chromatin filaments that connect them are not very visible. Basophils have receptors that can bind to IgE, IgG, complement, and histamine. The cytoplasm of basophils contains a varied amount of granules; these granules are usually numerous enough to partially conceal the nucleus. Granule contents of basophils are abundant with histamine, heparin, chondroitin sulfate, peroxidase, platelet-activating factor, and other substances.
When an infection occurs, mature basophils will be released from the bone marrow and travel to the site of infection.[21] When basophils are injured, they will release histamine, which contributes to the inflammatory response that helps fight invading organisms. Histamine causes dilation and increased permeability of capillaries close to the basophil. Injured basophils and other leukocytes will release another substance called prostaglandins that contributes to an increased blood flow to the site of infection. Both of these mechanisms allow blood-clotting elements to be delivered to the infected area (this begins the recovery process and blocks the travel of microbes to other parts of the body). Increased permeability of the inflamed tissue also allows for more phagocyte migration to the site of infection so that they can consume microbes.[18]